Synthesis of chemical probes
Materials
All chemicals and solvents were used as received from suppliers (Sigma-Aldrich (Merck), Thermo Fisher Scientific, Fluorochem or VWR) without further purification. Gases were from British Oxygen Company (BOC) Group and ultrapure water was used for all buffers.
Instrumentation
Hydrogen-1 nuclear magnetic resonance (1H-NMR) and carbon-13 nuclear magnetic resonance (13C-NMR) spectra were recorded on Bruker AV-400 (400 MHz) spectrometer, using residual isotopic solvent as an internal reference. Chemical shifts (δ) are given in units of parts per million (ppm). Each spectrum is corrected to the solvent reference signal. The multiplicity of each signal is given by singlet (s), doublet (d), triplet (t) or multiplet (m), and the number of protons (H) associated to a peak is indicated by nH. Coupling constants (J) are given in Hz and determined by analysis using MestReNova software.
Analytical LC–MS analysis was conducted on an Acquity UPLC BDH C18 column (50 mm × 2.1 mm, i.d. 1.7 µm packing diameter) at 40 °C. Flow rate was 0.5 ml min−1 and injection volume was 1 µl. The ultraviolet detection was a summed-up signal from wavelengths between 200 and 400 nm. UPLC retention times (tr) are reported in minutes. The following elution methods were used: method 1—(gradient of H2O and MeCN, supplemented with 0.1% formic acid) 3–100% MeCN for 0–1 min, 100% MeCN for 1–3.5 min, 100% to 3% MeCN for 3.5–3.6 min, 3% MeCN for 3.6–4 min; method 2—(gradient of 25 mM ammonium acetate (pH 8.0) and MeCN) 100% MeCN for 0–5 min, 100% MeCN for 5–5.5 min, 100% to 0% MeCN for 5.5–6.5 min, 0% MeCN for 6.5–9 min.
Chromatographic purifications were performed with a Biotage Isolera 4 using c-Hex/EtOAc gradient elution system. Final compounds were purified by PREP-LCMS (Agilent Technologies, 1260 series) equipped with a liquid chromatography/mass selective detector, an Agilent prep-C18 column (5 µm particle size, 21.2 × 50 mm) using water (containing 0.1% formic acid) and acetonitrile (containing 0.1% formic acid) in a gradient with a flow of 25 ml min−1.
Synthetic methods
Synthesis of compounds was performed according to Scheme 1 in Supplementary Information.
General method A (Jones oxidation)
The corresponding alcohol 1 (6.8 mmol) was dissolved in acetone (20 ml) and cooled to 0 °C, and 20 ml of chilled Jones reagent was added dropwise. The reaction was then allowed to warm up to room temperature and monitored by thin-layer chromatography (TLC) until completion. The reaction was quenched with 10% aqueous sodium thiosulfate, extracted with Et2O, dried and evaporated to give the target compound.
General method B (esterification)
The corresponding carboxylic acid (2a-c; 1 equiv.) was dissolved in MeOH (3 ml mmol−1) and heated to reflux. Concentrated H2SO4 (59 µl mmol−1) was added, and the reaction was monitored by TLC until completion. The reaction was then quenched with dH2O, extracted with Et2O, dried and evaporated to give compound 3 as a clear oil.
General method C (secondary amine formation)
The corresponding bromo methyl ester (3a-c; 1 equiv.) and propargylamine (10 equiv.) were dissolved in acetonitrile (30 ml g−1), and the reaction was set to reflux o/n and monitored by TLC. Upon completion, the solution was concentrated, cooled down and the resulting precipitate was collected by filtration, washed with cold acetonitrile and used in the next step without further purification.
General method D (amide bond formation)
The corresponding compound (4a-c; 1 equiv.) was dissolved in dry CH2Cl2 (3 ml mmol−1) under an inert argon atmosphere. N,N-Diisopropylethylamine (DIPEA) (3 equiv.) was added, and the reaction mixture was cooled to 0 °C. The corresponding acyl chloride was added (2 equiv.). The reaction was monitored until completion, upon which it was quenched with NaHCO3. The organic layer was extracted and dried, and the residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:1) to yield the target compound.
General method E (ester hydrolysis)
The corresponding compound (5a-i) was dissolved in THF (1.5 ml mmol−1) and treated dropwise with 1 M LiOH (5 equiv.). The reaction was monitored until completion, quenched via addition of 1 M HCl to pH 1, extracted with EtOAc, dried and evaporated to give the product usually in quantitative yield.
Methyl 12-bromododecanoate (3a)
A solution was prepared by dissolving 12-bromododecanoic acid (1.0 g, 3.58 mmol) in 12 ml of H2O. Then, 200 µl of H2SO4 was added, and the resulting solution was refluxed for 4 h. The reaction mixture was diluted with 50 ml Et2O. The layers were separated, and the organic solution was washed with NaHCO3 (aq.), H2O and brine before it was dried on Na2SO4, filtered and concentrated under reduced pressure to yield compound 3a (1.05 g, 3.4 mmol, 95%). 1H-NMR (400 MHz, chloroform-d) δ 3.66 (s, 3H), 3.40 (t, J = 6.9 Hz, 2H), 2.29 (t, J = 7.5 Hz, 2H), 1.90–1.78 (m, 2H), 1.65–1.56 (m, 2H), 1.46–1.35 (m, 2H), 1.34–1.24 (m, 12H). 13C-NMR (101 MHz, chloroform-d) δ 174.28, 51.41, 34.07, 34.01, 32.80, 29.41, 29.35, 29.19, 29.10, 28.71, 28.14 and 24.91.
Methyl 12-(prop-2-yn-1-ylamino)dodecanoate (4a)
Compound 3a (500 mg, 1.7 mmol) was dissolved in MeCN (10 ml). Propargylamine (140 mg, 2.55 mmol) and K2CO3 (469 mg, 3.4 mmol) were added, and the solution was stirred o/n at 85 °C. The reaction mixture was concentrated under reduced pressure, and the dried crude was dissolved in 50 ml EtOAc, washed with NaHCO3 (2×) and brine before it was dried on Na2SO4, filtered and concentrated under reduced pressure. The crude was purified by flash chromatography over silica gel using c-Hex/EtOAc (1:1) + 1% 7 N NH3 in MeOH to yield 4a (190 mg, 0.71 mmol, 42%). 1H-NMR (400 MHz, chloroform-d) δ 3.65 (s, 3H), 3.41 (d, J = 2.4 Hz, 2H), and 2.71–2.62 (m, 2H), 2.28 (t, J = 7.5 Hz, 2H), 2.19 (t, J = 2.4 Hz, 1H), 1.66–1.56 (m, 2H), 1.50–1.41 (m, 2H), 1.34–1.20 (m, 14H). 13C-NMR (101 MHz, chloroform-d) δ 174.25, 82.35, 71.07, 51.36, 48.71, 38.16, 34.09, 29.81, 29.51, 29.47, 29.38, 29.20, 29.11, 27.27 and 24.93.
Methyl 12-(N-(prop-2-yn-1-yl)acetamido)dodecanoate (5a)
Compound 4a (30 mg, 0.11 mmol) was dissolved in dry CH2Cl2 (2 ml). DIPEA (30 µl, 0.22 mmol) was added, and the solution was cooled on ice. Acetyl chloride (17 µl, 0.22 mmol) in 1 ml CH2Cl2 was added dropwise. The reaction mixture was stirred on ice for 4 h. The reaction was quenched with 5 ml NaHCO3 (aq.), extracted with EtOAc (3×) and the combined organic layers were dried on Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:1) to yield compound 5a (30 mg, 0.09 mmol, 86%). 1H- NMR (400 MHz, chloroform-d) δ 4.19 and 3.98 (d, J = 2.5 Hz, 2H), 3.65 (s, 3H), 3.38 (dt, J = 12.9, 7.5 Hz, 2H), 2.33–2.24 (m, 3H), 2.15 and 2.09 (s, 3H), 1.66–1.49 (m, 4H), 1.34–1.22 (m, 14H). 13C-NMR (101 MHz, chloroform-d) δ 174.25, 170.26, 170.00, 79.33, 72.29, 71.35, 51.39, 48.12, 46.27, 38.32, 34.09, 34.05, 29.47, 29.43, 29.36, 29.28, 29.20, 29.12, 28.42, 27.57, 26.88, 26.77, 24.94, 21.74 and 21.33.
12-(N-(Prop-2-yn-1-yl)acetamido)dodecanoic acid (6a; 16-Ac)
Compound 5a (15 mg, 0.048 mmol) was dissolved in THF (5 ml) and lithium hydroxide monohydrate (LiOH·H2O; 42 mg, 0.92 mmol) in H2O (100 µl) was added dropwise to the reaction mixture. The reaction mixture was stirred at room temperature for 4 h. The reaction was quenched with 5 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure. The crude product was purified by flash chromatography over silica gel using c-Hex/EtOAc (1:2→1:5) to yield compound 6a (14 mg, 0.048 mmol, quantitative). 1H-NMR (400 MHz, chloroform-d) δ 4.21 and 4.00 (d, J = 2.5 Hz, 2H), 3.40 (dt, J = 15.1, 7.6 Hz, 2H), 2.34 (t, J = 7.5 Hz, 2H), 2.28 and 2.18 (m, 1H), 2.18 and 2.12 (s, 2H), 2.20–2.15 (m, 4H), 1.38–1.24 (m, 14H). 13C-NMR (101 MHz, CDCl3) δ 178.41, 170.27, 79.24, 78.54, 77.32, 77.00, 76.68, 72.40, 71.43, 48.13, 46.36, 38.34, 34.10, 33.85, 29.42, 29.36, 29.29, 29.25, 29.20, 29.14, 28.98, 28.86, 28.38, 27.49, 26.74, 24.67, 21.68 and 21.29. In LC–MS method 1, the retention time was 1.53 min and the observed m/z calc. for C17H29NO3 (M + H)+ was 296.29, which closely matches the calculated value of 296.21.
Methyl 12-(N-(prop-2-yn-1-yl)cyclopropanecarboxamido)dodecanoate (5b)
Compound 4a (59 mg, 0.22 mmol) was dissolved in dry CH2Cl2 (2 ml). DIPEA (77 µl, 0.44 mmol) was added, and the solution was cooled on ice. Cyclopropanecarbonyl chloride (40 µl, 0.44 mmol) in 1 ml CH2Cl2 was added dropwise. The reaction mixture was stirred on ice for 4 h. The reaction was quenched with 5 ml NaHCO3 (aq.), extracted with EtOAc (3×) and the combined organic layers were dried on Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:1) to yield compound 5b (41 mg, 0.12 mmol, 55%). 1H-NMR (400 MHz, CDCl3) δ 4.19 (dd, J = 9.6, 2.6 Hz, 2H), 3.65 (s, 3H), 3.58–3.52 (m, 1H), 2.27 (d, J = 7.5 Hz, 2H), 1.72–1.50 (m, 5H), 1.26 (q, J = 8.0 Hz, 16H), 0.99 (qd, J = 5.3, 4.8, 1.8 Hz, 2H) and 0.77 (tt, J = 7.2, 3.3 Hz, 2H). 13C-NMR (101 MHz, CDCl3) δ 179.34, 174.34, 173.18, 79.58, 72.12, 71.28, 51.44, 47.42, 35.01, 34.09, 29.50, 29.45, 29.37, 29.32, 29.22, 29.12, 28.93, 26.85, 24.93, 20.47, 12.65, 11.20, 8.89, 7.97, 7.75 and 7.71.
12-(N-(Prop-2-yn-1-yl)cyclopropanecarboxamido)dodecanoic acid (6b; 16-cPr)
Compound 5b (41 mg, 0.12 mmol) was dissolved in THF (3 ml). In total, 610 µl of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature for 4 h. The reaction was quenched with 5 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure to yield compound 6b (13 mg, 0.040 mmol, 33%). 1H-NMR (400 MHz, CDCl3) δ 4.27–4.15 (m, 2H), 3.57 and 3.43 (t, J = 7.6 Hz, 1H), 2.34 (t, J = 7.5 Hz, 2H), 2.18 (d, J = 2.6 Hz, 1H), 1.83–1.51 (m, 5H), 1.40 – 1.18 (m, 14H), 1.02 (dt, J = 8.0, 4.2 Hz, 2H), 0.78 (dp, J = 7.2, 4.3 Hz, 2H). 13C-NMR (101 MHz, CDCl3) δ 178.96, 173.25, 79.58, 72.15, 71.30, 47.43, 37.61, 35.04, 33.93, 29.49, 29.41, 29.33, 29.29, 29.18, 29.01, 28.93, 27.62, 26.83, 24.68, 11.71, 11.24, 7.98 and 7.75. In LC–MS method 1, the retention time was 1.61 min and the observed m/z calc. for C19H31NO3 (M + H)+ was 322.33, which closely matches the calculated value of 322.23.
Methyl 12-(N-(prop-2-yn-1-yl)benzamido)dodecanoate (5c)
Compound 4a (59 mg, 0.22 mmol) was dissolved in dry CH2Cl2 (2 ml). DIPEA (77 µl, 0.44 mmol) was added and the solution was cooled on ice. Benzoyl chloride (51 µl, 0.44 mmol) in 1 ml CH2Cl2 was added dropwise. The reaction mixture was stirred on ice for 4 h. The reaction was quenched with 5 ml NaHCO3 (aq.), extracted with EtOAc (3×) and the combined organic layers were dried on Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:1) to yield compound 5c (43 mg, 0.12 mmol, 52%). 1H- NMR (400 MHz, CDCl3) δ 7.49–7.31 (m, 4H), 4.37 (s, 1H), 3.99 (s, 1H), 3.70 (s, 3H) 3.64 (s, 1H), 3.38 (s, 1H), 2.30 (td, J = 7.7, 2.6 Hz, 3H), 1.61 (tt, J = 8.0, 4.3 Hz, 4H), 1.41–1.02 (m, 14H). 13C-NMR (101 MHz, CDCl3) δ 174.28, 136.04, 129.69, 128.43, 126.78, 78.99, 51.41, 34.09, 29.44, 29.36, 29.21, 29.16, 29.12 and 24.93.
12-(N-(Prop-2-yn-1-yl)benzamido)dodecanoic acid (6c; 16-Bz)
Compound 5c (43 mg, 0.12 mmol) was dissolved in THF (3 ml). In total, 580 µl of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature for 4 h. The reaction was quenched with 5 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure to yield compound 6c (18 mg, 0.050 mmol, 44%). 1H-NMR (400 MHz, CDCl3) δ 7.49 (s, 1H), 7.41 (d, J = 3.9 Hz, 4H), 4.37 (s, 1H), 3.99 (s, 1H), 3.60 (s, 1H), 3.39 (s, 1H), 2.35 (t, J = 7.4 Hz, 2H), 2.29 (s, 1H), 1.64 (p, J = 7.3 Hz, 4H), 1.40 – 1.16 (m, 14H). In LC–MS method 1, the retention time was 1.66 min and the observed m/z calc. for C22H31NO3 (M + H)+ was 358.31, which closely matches the calculated value of 358.23.
Methyl 14-bromotetradecanoate (2b)
14-Bromododecan-1-ol (2.0 g, 6.8 mmol) was dissolved in acetone (50 ml). Jones reagent (10 ml) was added dropwise, and the reaction mixture was stirred for 2 h on ice. Half of the acetone was removed by rotary evaporation and H2O (50 ml) was added. The aqueous mixture was extracted with Et2O (3 × 75 ml). The combined organic layers were washed with H2O (2 × 25 ml), and brine before it was dried on Na2SO4 and concentrated under reduced pressure to yield compound 2a (2.1 g, 6.8 mmol, quantitative). 1H-NMR (400 MHz, chloroform-d) δ 3.40 (t, J = 6.9 Hz, 2H), 2.34 (t, J = 7.5 Hz, 2H), 1.85 (p, J = 7.0 Hz, 2H), 1.63 (q, J = 7.3 Hz, 2H), 1.43–1.26 (m, 20H). 13C-NMR (101 MHz, CDCl3) δ 180.15, 77.32, 77.00, 76.68, 34.40, 34.04, 33.95, 32.83, 29.52, 29.48, 29.40, 29.37, 29.19, 29.02, 28.74, 28.16, 25.00 and 24.64.
Methyl 14-bromotetradecanoate (3b)
Compound 2a (1.0 g, 3.4 mmol) was dissolved in dry MeOH (10 ml), H2SO4 (200 µl) was added and the mixture was stirred at 75 °C for 2 h. The mixture was then concentrated under reduced pressure until ~80% of the MeOH was evaporated. Water (50 ml) was added, and the aqueous mixture was extracted with Et2O (3×). The combined organic layers were washed with NaHCO3 (aq.), and brine before it was dried on Na2SO4, filtered and concentrated under reduced pressure to yield compound 3b (1.05 g, 3.4 mmol, quantitative). 1H-NMR (400 MHz, chloroform-d) δ 3.65 (s, 3H), 3.39 (t, J = 6.9 Hz, 2H), 2.28 (t, J = 7.5 Hz, 2H), 1.84 (dt, J = 14.4, 6.9 Hz, 2H), 1.61 (q, J = 7.4 Hz, 2H), 1.45–1.34 (m, 2H), 1.33–1.20 (m, 18H). 13C-NMR (101 MHz, CDCl3) δ 174.23, 77.32, 77.00, 76.68, 51.34, 34.06, 33.90, 32.81, 29.50, 29.46, 29.37, 29.19, 29.10, 28.71, 28.13, 25.71 and 24.91.
Methyl 14-(Prop-2-yn-1-ylamino)tetradecanoate (4b)
Compound 3b (0.5 g, 1.63 mmol) was dissolved in MeCN (15 ml), propargylamine (1.05 ml, 16.3 mmol) was added and the reaction mixture was stirred at 85 °C o/n. The solution was concentrated to ~4 ml, and the precipitate was collected by filtration. The filtrate was washed with cold MeCN to yield compound 4b (280 mg, 95 mmol, 58%). 1H-NMR (400 MHz, CDCl3) δ 3.86 (d, J = 2.6 Hz, 2H), 3.66 (s, 3H), 3.18–3.04 (m, 2H), 2.59 (t, J = 2.6 Hz, 1H), 2.30 (t, J = 7.6 Hz, 2H), 1.90 (p, J = 7.7 Hz, 2H), 1.61 (t, J = 7.3 Hz, 2H), 1.47–1.18 (m, 18H). 13C-NMR (101 MHz, CDCl3) δ 174.36, 78.44, 77.32, 77.00, 76.68, 72.43, 51.44, 46.26, 35.97, 34.11, 29.52, 29.46, 29.40, 29.35, 29.23, 29.13, 28.95, 26.75, 25.72 and 24.94.
14-(N-(Prop-2-yn-1-yl)acetamido)tetradecanoic acid (6d; 18-Ac)
Compound 4b (60 mg, 0.2 mmol) was dissolved in dry CH2Cl2 (5 ml). Acetyl chloride (21 µl, 0.3 mmol) was added, and the solution was cooled on ice. DIPEA (41 µl, 0.3 mmol) in CH2Cl2 (1 ml) was added dropwise. The reaction mixture was stirred for 2 h before NaHCO3 (5 ml) was added. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried on MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:3) to yield compound 5d (50 mg, 0.015 mmol, 75%). Compound 5d was used directly for ester hydrolysis without further purification.
Compound 5d (40 mg, 0. 11 mmol) was dissolved in THF (5 ml). In total, 1.2 ml of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature o/n. The reaction was quenched with 5 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (1:2→1:5) to yield compound 6d (25 mg, 0.077 mmol, 70%). 1H-NMR (400 MHz, CDCl3) δ 4.20 and 3.99 (d, J = 2.5 Hz, 2H), 3.46–3.34 (m, 2H), 2.33 (t, J = 7.5 Hz, 2H), 2.18 and 2.12 (m, 4H), 1.68–1.50 (m, 4H), 1.37–1.22 (m, 18H). 13C-NMR (101 MHz, CDCl3) δ 178.93, 178.84, 170.69, 170.35, 79.17, 78.61, 77.32, 77.00, 76.68, 72.41, 71.44, 48.14, 46.37, 38.34, 34.10, 33.98, 29.67, 29.50, 29.48, 29.45, 29.44, 29.39, 29.35, 29.29, 29.25, 29.19, 29.10, 29.02, 28.97, 28.34, 27.48, 26.81, 26.72, 24.69, 21.66 and 21.26. In LC–MS method 1, the retention time was 1.67 min and the observed m/z calc. for C19H33NO3 (M + H)+ was 324.27, which closely matches the calculated value of 324.26.
14-(N-(Prop-2-yn-1-yl)cyclopropanecarboxamido)tetradecanoic acid (6e; 18-cPr)
Compound 4b (40 mg, 0.13 mmol) was dissolved in dry CH2Cl2 (4 ml). Cyclopropanecarbonyl chloride (24 µl, 0.26 mmol) was added, and the solution was cooled on ice. DIPEA (45 µl, 0.26 mmol) in CH2Cl2 (1 ml) was added dropwise. The reaction mixture was stirred for 2 h before NaHCO3 (5 ml) was added. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried on MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using petroleum ether/EtOAc (2:1→1:3) to yield compound 5e (41 mg, 0.112 mmol, 92%). Compound 5e was used directly for ester hydrolysis without further purification.
Compound 5e (41 mg, 0.112 mmol) was dissolved in THF (5 ml). In total, 1.2 ml of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature o/n. The reaction was quenched with 5 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (1:2→1:5) to yield compound 6e (31 mg, 0.088 mmol, 68%). 1H-NMR (400 MHz, CDCl3) δ 4.22 and 4.19 (d, 2.6 Hz, 2H), 3.56 and 3.42 (t, J = 7.6 Hz, 2H), 2.33 (t, J = 7.5 Hz, 2H), 2.29 and 2.17 (s, 1H), 1.83–1.49 (m, 5H), 1.37–1.20 (m, 18H), 1.04–0.97 (m, 2H), 0.82–0.74 (m, 2H). 13C-NMR (101 MHz, CDCl3) δ 179.26, 173.29, 79.56, 77.35, 77.04, 76.72, 72.16, 71.31, 47.45, 47.11, 37.62, 35.05, 34.01, 29.56, 29.53, 29.51, 29.39, 29.33, 29.23, 29.15, 29.05, 28.93, 27.66, 26.86, 24.71, 11.71, 11.24, 8.00 and 7.77. In LC–MS method 1, the retention time was 1.72 min and the observed m/z calc. for C21H35NO3 (M + H)+ was 350.36, which closely matches the calculated value of found 350.26.
14-(N-(Prop-2-yn-1-yl)benzamido)tetradecanoic acid (6f; 18-Bz)
Compound 4b (85 mg, 0.29 mmol) was dissolved in dry CH2Cl2 (4 ml). Benzoyl chloride (31 µl, 0.29 mmol) was added, and the solution was cooled on ice. DIPEA (101 µl, 0.58 mmol) in CH2Cl2 (1 ml) was added dropwise. The reaction mixture was stirred for 2 h before NaHCO3 (5 ml) was added. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried on MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:3) to yield compound 5f (68 mg, 0.17 mmol, 59%). Compound 5f was used directly for the ester hydrolysis without further purification.
Compound 5f (40 mg, 0.112 mmol) was dissolved in THF (5 ml). In total, 1.2 ml of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature o/n. The reaction was quenched with 5 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (1:2→1:5) to yield compound 6f (28 mg, 0.072 mmol, 67%). 1H-NMR (400 MHz, CDCl3) δ 7.48 (s, 1H), 7.41 (s, 4H), 4.37 (s, 1H), 3.98 (s, 1H), 3.60 (s, 1H), 3.37 (s, 1H), 2.33 (t, J = 7.5 Hz, 2H), 2.28 (s, 1H), 1.72–1.50 (m, 4H), 1.43–1.10 (m, 18H). 13C-NMR (101 MHz, CDCl3) δ 179.40, 135.91, 129.70, 128.46, 126.81, 78.92, 72.42, 71.95, 34.05, 29.53, 29.52, 29.39, 29.21, 29.05 and 24.71. In LC–MS method 1, the retention time was 1.66 min and the observed m/z calc. for C24H35NO3 (M + H)+ was 386.35, which closely matches the calculated value of 386.26.
Methyl 16-(prop-2-yn-1-ylamino)hexadecanoate (4c)
Methyl 16-bromohexadecanoate (0.5 g, 1.43 mmol) was dissolved in MeCN (15 ml), propargylamine (916 µl, 14.3 mmol) was added and the reaction mixture was stirred at 85 °C o/n. The solution was concentrated, cooled down and the resulting precipitate was collected by filtration, then washed with cold MeCN to yield compound 4c (391 mg, 1.21 mmol, 85%). 1H-NMR (400 MHz, CDCl3) δ 3.86 (d, J = 2.6 Hz, 2H), 3.67 (s, 3H), 3.17–3.09 (m, 2H), 2.59 (t, J = 2.6 Hz, 1H), 2.30 (t, J = 7.6 Hz, 2H), 1.89 (q, J = 7.9 Hz, 2H), 1.61 (d, J = 7.4 Hz, 2H), 1.41 (t, J = 7.7 Hz, 2H), 1.35–1.23 (m, 22H). 13C-NMR (101 MHz, CDCl3) δ 174.39, 78.38, 72.54, 51.46, 46.31, 35.99, 34.14, 29.63, 29.59, 29.51, 29.45, 29.39, 29.27, 29.17, 29.00, 26.79, 25.81 and 24.97.
16-(N-(Prop-2-yn-1-yl)acetamido)hexadecanoic acid (6g; 20-Ac)
Compound 4c (50 mg, 0.15 mmol) was dissolved in dry CH2Cl2 (3 ml). Acetyl chloride (21 µl, 0.3 mmol) was added, and the solution was cooled on ice. DIPEA (54 µl, 0.3 mmol) in CH2Cl2 (1 ml) was added dropwise. The reaction mixture was stirred for 2 h before NaHCO3 (5 ml) was added. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried on MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:3) to yield compound 5g (10 mg, 0.027 mmol, 18%).
Compound 5g (10 mg, 0.027 mmol) was dissolved in THF (1 ml). In total, 140 µl of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature for 4 h. The reaction was quenched with 1 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure to yield compound 6g (6 mg, 0.017 mmol, 60%). 1H-NMR (400 MHz, CDCl3) δ 4.21 (d, J = 2.5 Hz, 2H), 4.00 (d, J = 2.5 Hz, 1H), 3.41–3.34 (m, 2H), 2.18 (d, J = 1.9 Hz, 2H), 2.12 (s, 3H), 1.62 (q, J = 7.3 Hz, 4H), 1.26 (d, J = 2.9 Hz, 22H). 13C-NMR (101 MHz, CDCl3) δ 178.09, 169.78, 72.40, 71.43, 48.15, 38.36, 34.09, 29.57, 29.28, 29.22, 26.74, 24.72, 21.33. In LC–MS method 1, the retention time was 1.61 min and the observed m/z calc. for C21H37NO3 (M + H)+ was 352.36, which closely matches the calculated value of 352.28.
Methyl 16-(N-(prop-2-yn-1-yl)cyclopropanecarboxamido)hexadecanoate (5h)
Compound 4c (90 mg, 0.28 mmol) was dissolved in dry CH2Cl2 (5 ml). Cyclopropanecarbonyl chloride (50 µl, 0.56 mmol) was added, and the solution was cooled on ice. DIPEA (97 µl, 0.56 mmol) in CH2Cl2 (1 ml) was added dropwise. The reaction mixture was stirred for 2 h before NaHCO3 (5 ml) was added. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried on MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:3) to yield compound 5h (53 mg, 0.14 mmol, 49%). 1H-NMR (400 MHz, CDCl3) δ 4.26–4.19 (m, 2H), 3.68 (s, 3H), 3.57 (d, J = 7.8 Hz, 1H), 2.32 (t, J = 7.5 Hz, 2H), 1.77–1.56 (m, 5H), 1.29 (d, J = 11.8 Hz, 23H), 1.03 (dq, J = 8.5, 4.5, 3.7 Hz, 2H), 0.80 (dt, J = 7.8, 3.4 Hz, 2H). 13C-NMR (101 MHz, CDCl3) δ 174.36, 173.13, 79.64, 71.24, 51.45, 47.41, 34.98, 34.13, 29.64, 29.59, 29.57, 29.45, 29.35, 29.26, 29.16, 28.95, 26.87, 24.97, 11.20, 7.96 and 7.70.
16-(N-(Prop-2-yn-1-yl)cyclopropanecarboxamido)hexadecanoic acid (6h; 20-cPr)
Compound 5h (53 mg, 0.14 mmol) was dissolved in THF (3 ml). In total, 700 µl of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature for 4 h. The reaction was quenched with 1 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure to yield compound 6h (32 mg, 0.085 mmol, 61%). 1H-NMR (400 MHz, CDCl3) δ 4.21 (dd, J = 11.6, 2.5 Hz, 2H), 3.60–3.51 (m, 1H), 3.43 (t, J = 7.6 Hz, 1H), 2.34 (t, J = 7.5 Hz, 2H), 2.18 (t, J = 2.5 Hz, 1H), 1.85–1.56 (m, 5H), 1.36–1.24 (m, 22H), 1.01 (td, J = 6.3, 5.4, 2.7 Hz, 2H), 0.78 (dt, J = 7.9, 3.4 Hz, 2H). 13C-NMR (101 MHz, CDCl3) δ 178.88, 173.24, 79.59, 72.13, 71.29, 47.44, 35.03, 33.92, 29.61, 29.54, 29.42, 29.34, 29.24, 29.06, 28.93, 26.86, 24.71, 11.24, 8.00 and 7.76. In LC–MS method 1, the retention time was 1.61 min and the observed m/z calc. for C23H39NO3 (M + H)+ was 378.40, which closely matches the calculated value of 378.29.
16-(N-(Prop-2-yn-1-yl)benzamido)hexadecanoic acid (6i; 20-Bz)
Compound 4c (70 mg, 0.22 mmol) was dissolved in dry CH2Cl2 (2 ml). Benzoyl chloride (50 µl, 0.44 mmol) was added, and the solution was cooled on ice. DIPEA (76 µl, 0.44 mmol) in CH2Cl2 (1 ml) was added dropwise. The reaction mixture was stirred for 2 h before NaHCO3 (5 ml) was added. The layers were separated, and the aqueous layer was extracted with CH2Cl2. The combined organic layers were washed with brine, dried on MgSO4 and concentrated under reduced pressure. The residue was purified by flash chromatography over silica gel using c-Hex/EtOAc (2:1→1:3) to yield compound 5i (58 mg, 0.14 mmol, 63%). Compound 5i (40 mg, 0.094 mmol) was dissolved in THF (3 ml). In total, 470 µl of a 1 M LiOH solution in H2O was added dropwise. The reaction mixture was stirred at room temperature for 4 h. The reaction was quenched with 1 ml HCl (1 M), extracted with EtOAc (3×) and the combined organic layers were washed with brine, dried on Na2SO4 and concentrated under reduced pressure to yield compound 6i (12 mg, 0.029 mmol, 31%). 1H-NMR (400 MHz, CDCl3) δ 7.48 (s, 1H), 7.41 (d, J = 3.6 Hz, 4H), 4.37 (s, 1H), 3.99 (s, 1H), 3.61 (s, 1H), 3.37 (s, 1H), 2.33 (t, J = 7.5 Hz, 2H), 2.28 (s, 1H), 1.62 (p, J = 7.4 Hz, 4H), 1.30–1.14 (m, 20H). 13C-NMR (101 MHz, CDCl3) δ 179.62, 136.39, 130.20, 128.90, 127.25, 79.42, 72.26, 34.43, 30.05, 30.03, 29.99, 29.85, 29.67, 29.50 and 25.16. In LC–MS method 1, the retention time was 1.86 min and observed m/z calc. for C26H39NO3 (M + H)+ was 414.34, which closely matches the calculated value of 414.29.
C18-Bz-CoA probe
To a suspension of 14-(N-(prop-2-yn-1-yl)benzamido)tetradecanoic acid (30 mg, 78 μmol, 2 equiv.) in dry THF (1.2 ml) was added a solution of 1,1′-carbonyl-diimidazole (15 mg, 94 μmol, 2.4 equiv.) in CH2Cl2 (1.2 ml), under nitrogen atmosphere. The clear reaction mixture was stirred for 45 min at room temperature. The reaction mixture was concentrated under reduced pressure. The residue was dissolved in dry THF (1.2 ml). Coenzyme A hydrate (30 mg, 39 μmol, 1 equiv.) was dissolved in an aqueous solution of NaHCO3 (0.5 M, 4 ml) and added dropwise to the solution of activated acid. The reaction mixture was stirred at room temperature for 3 h under nitrogen atmosphere, flash frozen in liquid N2 and lyophilized overnight. The samples were then dissolved in 1 ml H2O, and the product was purified by preparative RP-HPLC over a gradient of 25 mM ammonium acetate pH 8 in MeCN. C18-Bz-CoA 19 was obtained as a white lyophilized solid (22 mg, 47% yield). 1H-NMR (400 MHz, D2O) δ 8.51 (s, 1H), 8.20 (s, 1H), 7.35 (dd, J = 13.8, 7.6 Hz, 4H), 7.27 (d, J = 7.4 Hz, 1H), 6.08 (d, J = 6.1 Hz, 1H), 4.80–4.70 (m, 2H), 4.53 (p, J = 2.8 Hz, 1H), 4.23–4.16 (m, 2H), 3.98 (s, 1H), 3.84 (dd, J = 9.8, 5.0 Hz, 1H), 3.52 (dd, J = 9.8, 4.7 Hz, 1H), 3.37 (p, J = 6.7 Hz, 2H), 3.22 (dtd, J = 11.1, 7.8, 7.3, 3.4 Hz, 4H), 2.89 (t, J = 6.7 Hz, 2H), 2.64 (d, J = 2.5 Hz, 1H), 2.42 (t, J = 7.4 Hz, 2H), 2.35 (t, J = 6.9 Hz, 2H), 1.44 (p, J = 7.3 Hz, 4H), 1.14–0.91 (m, 18H), 0.87 (s, 3H), 0.70 (s, 3H). 13C-NMR (151 MHz, D2O) δ 174.65, 173.41, 148.71, 140.74, 134.73, 128.69, 126.36, 118.34, 86.96, 82.43, 78.89, 74.06, 73.91, 65.16, 50.96, 43.67, 38.72, 38.44, 38.39, 35.47, 35.37, 29.32, 29.16, 28.98, 28.57, 27.99, 25.36, 21.05 and 18.13. In LC–MS method 2, the retention time was 4.39 min and observed m/z calc. for C45H69N8O18P3S (M + H) + was 1135.5, which closely matches the calculated value of 1135.37.
Cell culture and compound preparation
HEK293T, HEK293-FT, MDA-MB-231 and PANC1 cell lines were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with GlutaMAX (Thermo Fisher Scientific, 10566016), 10% vol/vol FBS, 100 U ml−1 penicillin and 0.1 mg ml−1 streptomycin (Thermo Fisher Scientific, 15140122) in a 37 °C, 5% CO2 incubator. Cells were selected with puromycin dihydrochloride (Thermo Fisher Scientific, A1113803), blasticidin S hydrochloride (Thermo Fisher Scientific, A1113903) and hygromycin B (Thermo Fisher Scientific, 10687010) at final concentrations noted below. Synthetic lipid-based loading and transfer probes and alkyne palmitic acid (2BScientific, BCAL-015-25), palmostatin B (Sigma-Aldrich, 178501), TAMRA or biotin PEG3 azide (Sigma-Aldrich, 760757 or 762024), tris((1-benzyl-1H-1,2,3-triazol-4-yl)methyl)amine (TBTA; Sigma-Aldrich, 678937) and palmitoyl coenzyme A (Sigma-Aldrich, P9716; ≥90%) were dissolved in DMSO and stored at −20 °C. Sealed ampules containing a 0.5 M aqueous solution of tris (2-carboxyethyl) phosphine (TCEP) and TCEP HCl (C4706) were purchased from Sigma-Aldrich, and n-dodecyl-β-d-maltopyranoside (DDM) was purchased from Generon (D310LA). TCEP HCl was prepared fresh as 50 mM stock in Milli-Q H2O, DDM was prepared as a 10% stock solution in Milli-Q H2O and stored at −20 °C and cOmplete, EDTA-free protease inhibitor cocktail tablets were used according to the manufacturer’s instructions (Sigma-Aldrich, 11873580001).
Antibodies and western analysis
Mouse-derived monoclonal antibodies for FLAG M2 (F1804), HA-epitope (HA-7, H3663) and α-tubulin (T5168) and rabbit-derived polyclonal antibodies for ZDHHC20 (Atlas Antibodies, HPA014702), BCAP31 (Atlas Antibodies, HPA003906) and V5-epitope (SAB1306079) were purchased from Sigma-Aldrich. Mouse monoclonal anti-GFP (GF28R) antibody was purchased from Generon, rabbit monoclonal anti-TOMM20 (ab186735) antibody was purchased from Abcam, rabbit polyclonal anti-TMX1 (HPA003085) antibody was purchased from Atlas Antibodies, rabbit polyclonal anti-NCAM1/CD56 (14255-1-AP) and anti-PI4K2A (15318-1-AP) antibodies were bought from ProteinTech and rabbit-derived polyclonal antibodies against vinculin (42H89L44) and calnexin (ab22595) were purchased from Thermo Fisher Scientific and Abcam, respectively. Secondary antibodies were horseradish peroxidase (HRP)-conjugated, polyclonal goat-derived antibodies against mouse (Dako, P0447) and rabbit (Dako, P0448), or fluorophore-conjugated IRDye 680CW goat anti-mouse (Licor, 926-68072) and IRDye 680CW goat anti-rabbit (Licor, 926-32211). Western blot analysis was accomplished through SDS–PAGE of cell lysates or affinity-resin eluates in 1× Laemmli loading buffer (Bio-Rad, 1610747) containing 2.5% β-mercaptoethanol and transfer of protein onto polyvinylidene fluoride (PVDF) or nitrocellulose using the Trans-Blot Turbo System (Bio-Rad). Secondary HRP-conjugates were visualized after addition of Clarity Western ECL Substrate (Bio-Rad, 1705061) and chemiluminescent detection in an Amersham Imager 680 (GE Life Sciences) or fluorescence detection using a LICOR Odyssey CLx. Quantitation of western blot protein intensity was performed by densitometry using ImageJ 1.50c or Image Studio Lite (GE Life Sciences), and data were plotted using Prism 9.0.
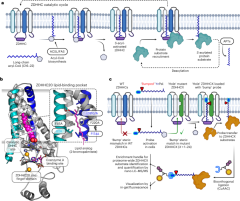
ZDHHC structural modeling
Human ZDHHC family protein sequences were aligned using the ‘Create Alignment’ module of CLC Sequence Viewer 7. Regions of sequence similarity that also overlap with ZDHHC20 transmembrane helices (TMs) 1–4 and the DHHC-containing cysteine-rich domain were identified and selected for homology modeling. To generate homology models for ZDHHCs 1–19 and 21–24, selected sequences were individually submitted to the Protein Homology/analogY Recognition Engine V 2.0 (Phyre2) using the ‘Normal’ modeling mode55. To identify putative bump-hole mutations, homology models were structurally aligned to the ZDHHC20-2BP crystal structure (Protein Data Bank (PDB) ID: 6BML) using MacPyMOL: PyMOL V1.5.0.4. Residues located on TM3 and spatially overlapping with or proximal to ZDHHC20-Y181 were prioritized for bump-hole analysis; however, several ZDHHC models did not present residues meeting these criteria. In this case, strict rules were adopted including (1) selection of ZDHHC20-Y181 or (2) -F65 proximal residues located on TMs 2 and 3 overlapping with ZDHHC20 residues having B-factor values <100 and (3) when rules 1 and 2 fail, residues on TM1 proximal to the ω-position of the 2BP fatty acid chain and with lowest ZDHHC20 B-factor value were selected for bump-hole screening. Residues presented by TM1 were given least priority as their side chains have access to the lipid-binding pocket and the bilayer. Potentially, mutations on this helix could generate a hole in the pocket, leading to structural instability or loss of lipid-probe binding affinity.
Molecular biology and cloning
Plasmids and subcloning
For a complete list of vectors used in this study, refer to Supplementary Table 2. The preparation of new plasmids generated for this study will be described herein. Human C-terminally Myc-FLAG-tagged ZDHHC20 (C-FLAG-D20) was purchased from Origene Technologies (MR205665). Plasmids for expression of N-terminally 3xFLAG-tagged human ZDHHCs 1–24 (N-FLAG-DX, X = 1–24), HA-tagged GCP16 (GOLGA7, ZDHHC9 cofactor) and empty pEF-1α vector were a generous gift from Y. Ohno (Hokkaido University). The C-FLAG-pcDNA3 expression vector (Addgene, 20011) was also used as a negative control for FLAG-tagged ZDHHC expression. C-terminally Myc-HA-tagged ZDHHC20 (C-HA-D20) PCR fragment was subcloned into the PmeI and AsiSI linearized C-FLAG-D20 vector using the NEBuilder HiFi Assembly Kit (NEB, E5520S). C-HA-D20 fragment, with HA-tag sequence spacer (bold), was generated by PCR using Phusion DNA Polymerase and the primer set in Supplementary Table 3. C-FLAG-D20 was subcloned into the pLVX-TetOne-Puro (Clontech) and in-house attb vectors, expressing mouse ZDHHC20 and containing a blasticidin resistance marker (attb-ZDHHC20-BSDr), using the same strategy. Empty pLVX-TetOne-Puro and attb-ZDHHC20-BSDr were linearized using EcoRI and BamHI and XhoI and MfeI, respectively, and human C-FLAG-D20 fragments generated with primer sets indicated in Supplementary Table 3. V5-tagged TurboID (promiscuous BirA mutant, V5-Turbo-NES-pCDNA3; Addgene, 107169) and C-FLAG-D20 or EGFP (pEGFP-N1-FLAG; Addgene, 60360) chimeras were also subcloned into the XhoI- and MfeI-linearized attb-BSDr vector using the NEBuilder HiFi Assembly Kit, and C-FLAG-D20 WT and C-FLAG-D20(Y181G) were subcloned into the BamHI- and NotI-linearized pcDNA5/FRT (see Supplementary Table 4 for primer sets). Plasmids for the expression of PI4K2A (pDONR223-PI4K2A; Addgene, 23503), TOMM20 (mCherry-TOMM20-N-10; Addgene, 55146) and TFAM (pcDNA3-TFAM-mCLOVER; Addgene, 129574) were subcloned into the EcoRI- and XhoI-linearized N-HA-Ifitm3 vector using the NEBuilder HiFi Assembly Kit (see Supplementary Table 3 for primer sets used to create N-HA-tagged plasmids). pcDNA3.1 XXYLT1-HA was kindly provided by H. Bakker. All new plasmids were se